Antigen Selection
Our labs could raise antibodies against full-length proteins, soluble fragments (>30 residues) or peptide antigens. In the latter two cases, we consider the choice of antigens a key determinant of antibody quality. Using expertise in both sequence analysis and structural biology, our scientists have developed a proprietary algorithm, AccuTope, for the identification of suitable peptide or fragment antigens. The software evaluates potential antigens by the following criteria:
Uniqueness of sequence -- Which would ensure the specificity of the antibodies raised.
Solvent accessibility -- An important consideration for antibodies used under native or partially denaturing conditions (e.g., in IP, CHIP, IF or IHC).A good antigen (or epitope) should be accessible to the antibody in the native protein.
Consistence of Conformation -- A good antigen should assume the same conformation as a peptide (or fragment) and as a part of the native protein. Antibodies raised against such an antigen would recognize the native protein. Two types of antigens fulfill this criterion:
a) Disordered fragments (i.e., flexible loops lacking any secondary structure)b) Autonomously folded fragments (whose conformation do not depend on the rest of the native protein).
The AccuTope program is designed to recognize both types.
Antigen Presentation
For immunization, an antigen could be displayed with VLP, KHL or both, depending on customer preference and technical feasibility. Our labs could either crosslink the antigen to the enhancement factor (EF) directly, or express an antigen-EF fusion protein.
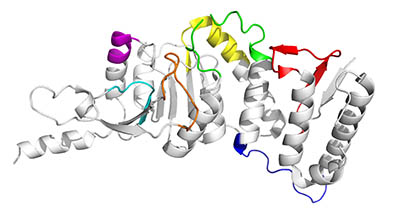
Fig 1. The Selection of Antigens with the AccuTope Algorithm. The structure of human DOT1L histone-lysine N-methyltransferase is shown as grey ribbons. Fragments predicted as good peptide antigens are shown in colors.
